Describe the Electron Cloud Model of the Atom
As such the atom is the basic building block of chemistry. In this module students reconnect with and deepen their understanding of statistics and probability concepts first introduced in Grades 6 7 and 8.

Modern Atomic Theory The Scientist Who Developed Atomic Theory Atomic Theory Modern Atomic Model Atom
One ampere of current represents one coulomb of electrical charge 624 x 10 18 charge carriers moving past a specific point in one second.

. The rest mass of the electron is 91093837015 1031 kg which is only 11836the mass of a proton. The standard unit is the ampere symbolized by A. The electron is a subatomic particle denoted by the symbol e or β whose electric charge is negative one elementary charge.
Lorentz Dispersion Model Spectroscopic ellipsometry SE is a technique based on the measurement of the relative phase change of reflected and polarized light in order to characterize thin film optical functions and other properties. Access the answers to hundreds of Bohr model questions that are explained in. Electron lightest stable subatomic particle known.
This model can be portrayed as a nucleus surrounded by an electron cloud. It has a special place in history because it introduced the quantum theory which gave rise to quantum mechanics. Most of the atom is empty space.
Understanding the atom lesson 1 answer key. Thompson by the end of the 19th century was a crucial step in the development of atomic physics. Bohr Model Questions and Answers.
Atom smallest unit into which matter can be divided without the release of electrically charged particles. It carries a negative charge of 1602176634 1019 coulomb which is considered the basic unit of electric charge. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons.
The electron has a mass that is approximately 11836 that of the. The Bohr Model of the Hydrogen Atom attempts to fill in some of the gaps left by Rutherfords model. The rest consists of a positively charged nucleus of protons and neutrons surrounded.
It also is the smallest unit of matter that has the characteristic properties of a chemical element. An electron is therefore considered nearly massless in comparison with a proton or a neutron and the. Current is a flow of electrical charge carriers usually electrons or electron-deficient atoms.
Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons. The Plum Pudding Model which was devised by JJ. This atomic model is known as the quantum mechanical model of the atom.
In this electron configuration we have a bunch of letters and numbers raised to different powers. Algebra I Module 2. Unlike the Bohr model the quantum mechanical model does not define the exact path of an electron but rather predicts the odds of the location of the electron.
An X-ray crystallographic experiment produces an electron density map for the average unit cell of the protein crystalThe amino acid or nucleotide sequence of the crystallized polymers is known in advance. The measured data are used to describe a model where each layer refers to a given ma-terial. Get help with your Bohr model homework.
Each letter and number pair represents a group of electron orbitals in the atom. Crystallography Produces Electron Density Maps. The common symbol for current is the uppercase letter I.
The crystallographer fits the atoms of the known molecules into the electron density map and. Electrons belong to the first generation of the lepton particle family and are generally thought to be elementary particles because they have no known components or substructure. In comparison to the valence shell atom model the Bohr model is a more rudimentary representation of the hydrogen atom.

The Electron Cloud Model Explained Animation Atomic Orbitals Tutorial Crash Chemistry Academy Youtube Chemistry Worksheets Chemistry Help Astrophysics

Electron Cloud Theory Explained Electrons Clouds Universe Today

Atomic Structure Parts Of The Atom Bundle Sharing Lessons Middle School Lessons Education Lesson Plans

Bohr Vs Electron Cloud Electrons Bohr Model Clouds

Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science Science Lessons Science Lesson Plans Kesler Science

Electron Cloud Easy Science Electrons Physical Change Chemical Changes

Atomic Structure Vocabulary Card Sort Teaching Chemistry Chemistry Classroom Chemistry Lessons

Electron Configurations Describe Where Electrons Are Located Around The Nucleus Of An Atom For Example The Electron Configuration Electrons Atomic Structure

Next Upper Atomic Model Science Education Chemistry Lessons Chemistry Classroom Teaching Chemistry

Elecrtonegativity Of Elements Is Directly Correlated To The Size Of Their Electron Cloud Molecules Organic Chemistry Study Chemistry
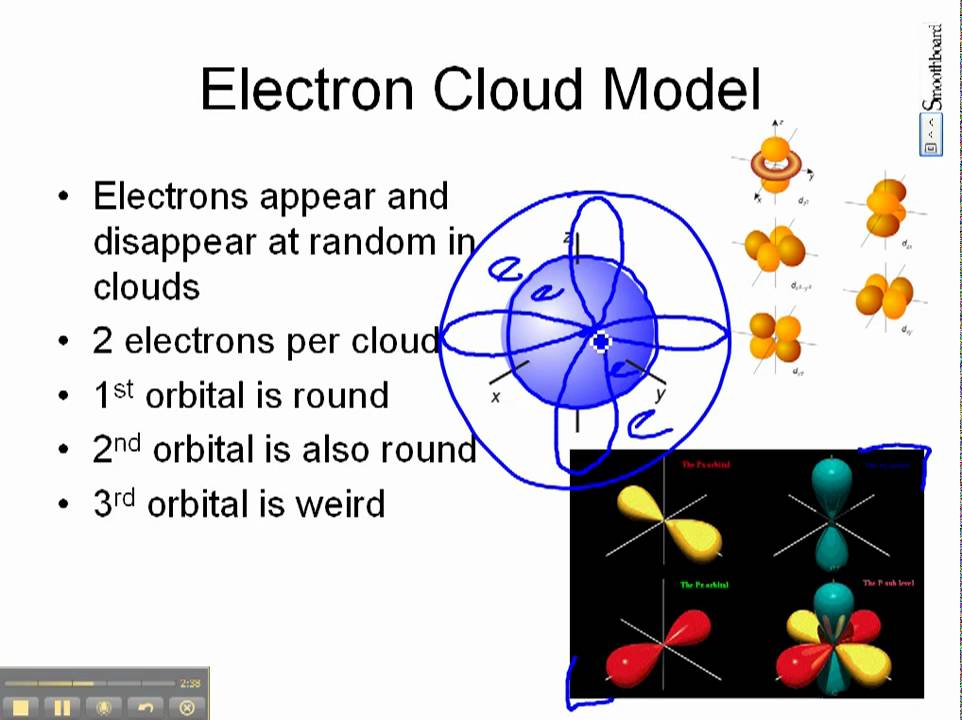
Electron Cloud Model Electrons Elementary Science Clouds

What Is This Something In Outer Space A Galaxy Perhaps Nope This Is An Atom The Atom Can Be Referred T Quantum Physics Atomic Theory Physics Mechanics

The Quantum Mechanical Model Of The Atom Article Khan Academy Mechanical Model Quantum Electrons

Niels Bohr Atomic Theory And Its Limitations Atomic Theory Niels Bohr Chemistry Lessons

The Electrons Are On The Outside In The Electron Cloud Normally They Are The Same Count As The Proton To Balan Electrons Science Chemistry Chemical Equation

Atomic Structure Parts Of The Atom Bundle Atomic Structure Atom Science Lessons

See The Electron Configuration Diagrams For Atoms Of The Elements Atom Diagram Electron Configuration Atom Project

Atomic Structure Periodic Table Chemistry Classroom Chemistry Class Chemistry Education

Worldofchemicals On Twitter Atomic Theory Atom Diagram Atom Model
Comments
Post a Comment